Objectives:
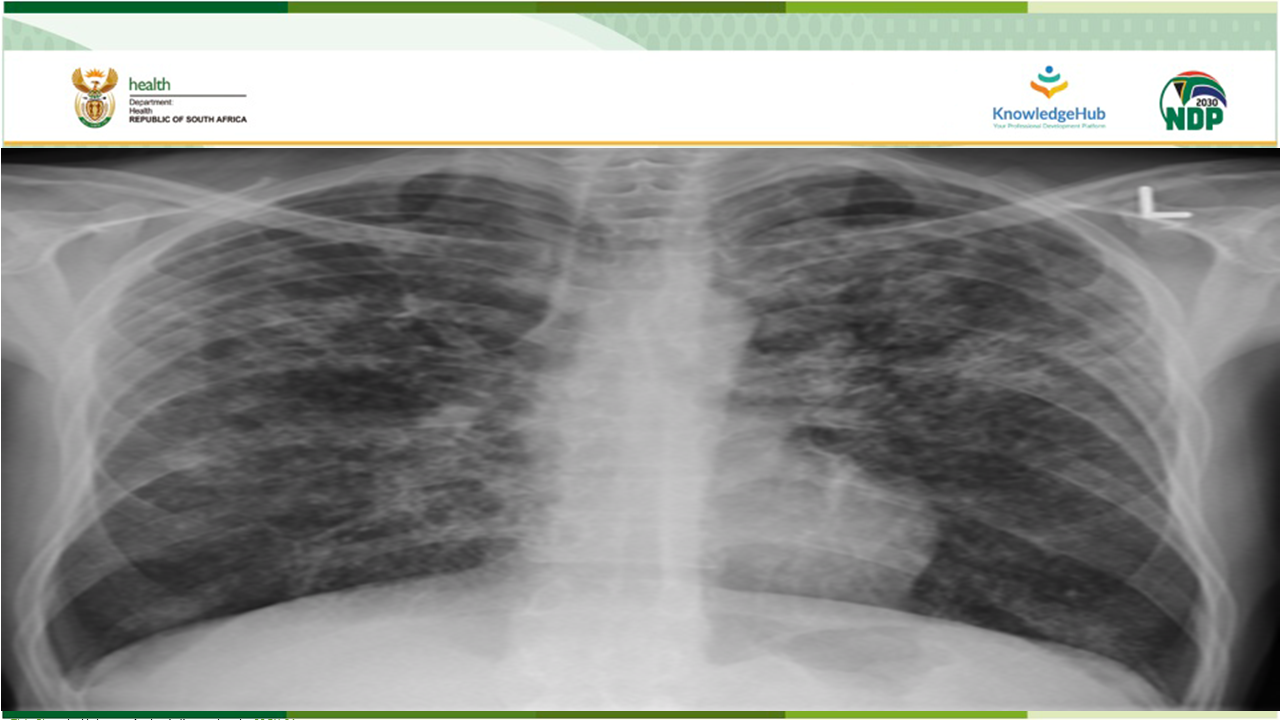
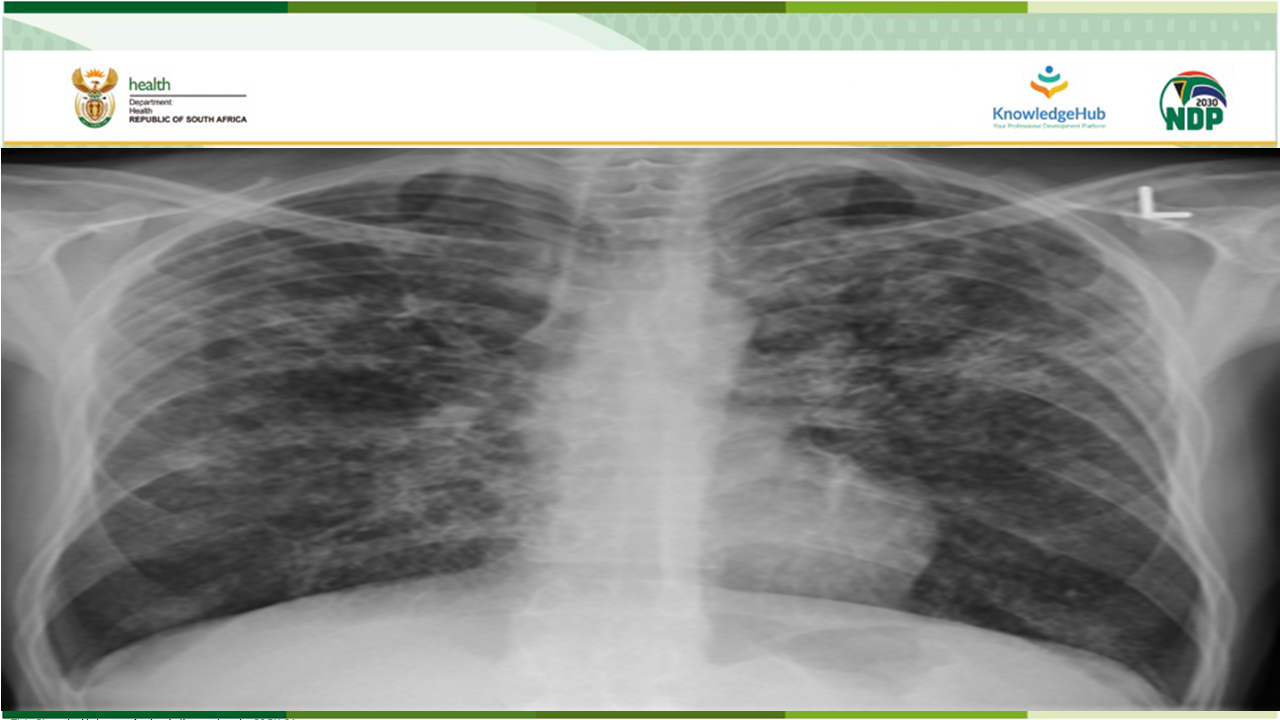
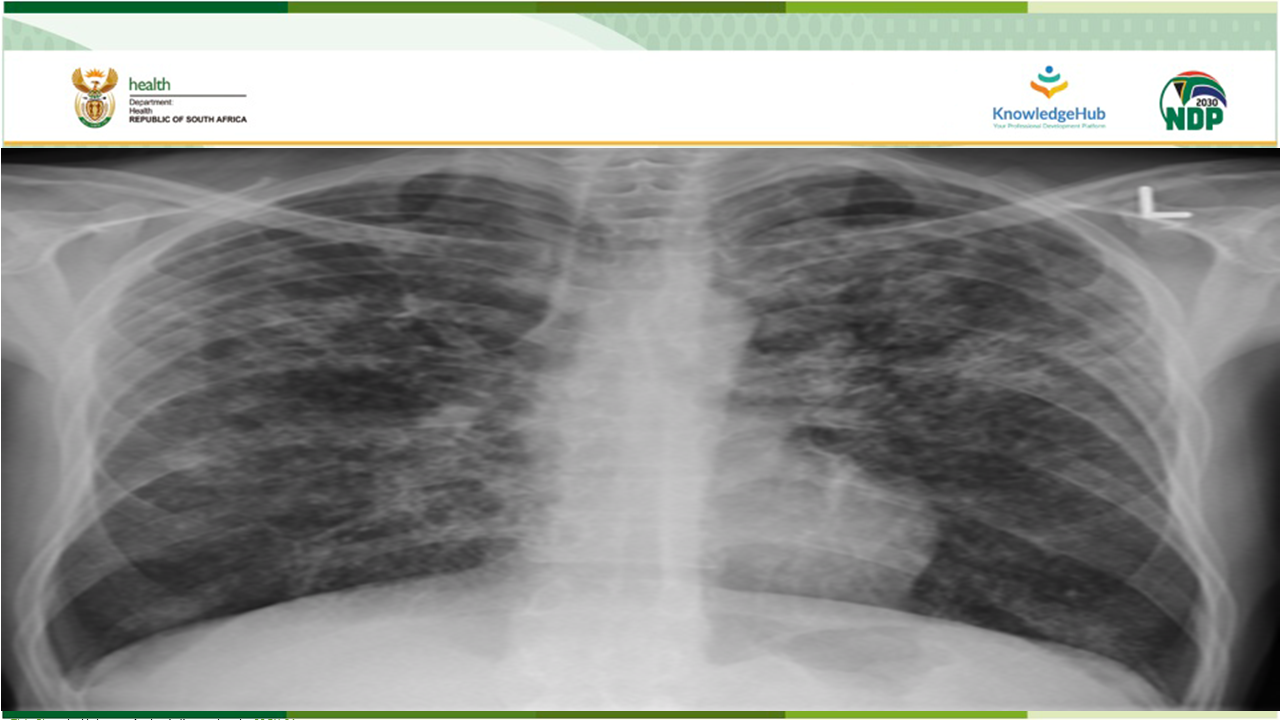
South Africa has been a global leader in introducing innovation to the field of Rifampicin-resistant tuberculosis (RR-TB) and the work done in the country has had a significant impact on global policy. One of these innovations are the implementation of clinical access programmes for Bedaquiline, followed by Delamanid and BPaL.
In May 2022, the World Health Organization (WHO) announced that the six-month BPaL/M regimen, comprising of bedaquiline, pretomanid, linezolid and moxifloxacin, which may be used programmatically in the place of the nine-month or longer 18-month drug resistant tuberculosis (DR-TB) regimen in patients with RR-TB.
After a robust GRADE review (Adolopment) of this Global Recommendation, the South African National Essential Medicine Committee (NEMLC) recommended that the country may use BPaL-L, a regimen made of bedaquiline, pretomanid, linezolid and levofloxacin.
This webinar will discuss the implementation and roll-out of this new six-month BPaL-L RR-TB regimen.
Discuss NDOH plan for introduction of BPaL-L.
Discuss major changes to RR-TB guidelines regarding: diagnosis, eligibility criteria for treatment, treatment regimen, patients’ follow-up.
Speakers:
Prof Norbert Ndjeka
Chief Director: TB Control and Management Cluster (NDoH)
Qualifications: MD, DHSM (Wits), MMed (Fam Med) (MED), Dip HIV Man (CMSA), DSc (h.c.)
Dr Francesca Conradie
Deputy Executive Director: Wits Health Consortium
Qualifications: MBChB
Dr Lindiwe Mvusi
Director DS-TB (NDoH)
Qualifications: MBChB
Video: